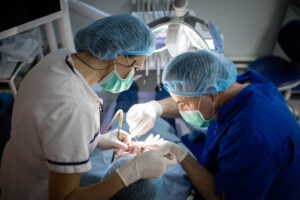
Exploring Periodontal Surgery: When Is It Necessary?
Periodontal health is essential for keeping your smile strong, comfortable, and functional. While early stages of gum disease can often be treated with non-surgical therapies such as scaling and root planing, more advanced cases may require surgical intervention to...
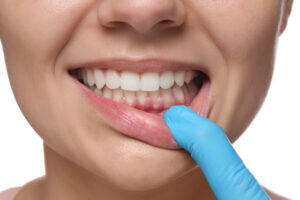
Effective Bleeding Gums Treatment: Regain Healthy Gums Today
Bleeding gums are a common oral health concern, but many people dismiss them as a minor issue. In reality, bleeding gums can signal the early stages of gum disease or other underlying problems that require professional care. If you notice bleeding while brushing,...
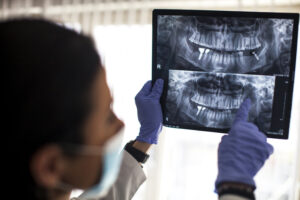
Understanding Implant Dentistry: How an Implant Dentist Can Restore Your Smile
A healthy smile is about much more than appearances. It influences how you feel about yourself, how comfortable you are in social settings, and how well you can perform everyday functions like speaking and chewing. When tooth loss happens, whether from gum disease,...
The Benefits of Dental Implants: A Permanent Solution to Tooth Loss
Tooth loss can have a profound effect on your daily life, your health, and your confidence. For many people, losing even a single tooth can cause difficulty eating, trouble speaking clearly, and a noticeable change in appearance. Over time, missing teeth can also...
Why You Should See a Periodontist for Your Gum Health
When most people think about oral health, they think about brushing, flossing, and seeing their dentist for regular checkups to prevent cavities. While these are crucial steps, many people overlook one essential component of oral health: their gums. Healthy gums...
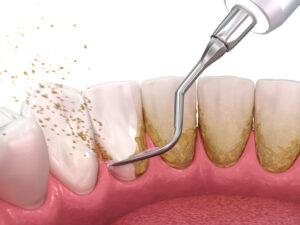
Deep Cleaning Demystified: What Happens During Scaling and Root Planing?
Gum disease rarely announces itself with a dramatic flare-up. It usually begins with subtle inflammation, bleeding during brushing, or minor tenderness. Left unchecked, that irritation can advance—wearing away the tissue and bone that keep teeth stable. Scaling and...
Periodontal Maintenance vs. Regular Cleanings: What’s the Real Difference?
Maintaining a healthy smile takes more than diligent brushing and flossing. You also need professional care that matches your individual gum health. For many patients, twice-yearly dental cleanings are the gold standard—yet if you’ve ever been diagnosed with gum...
Dental Implants Help Patients with Ill-Fitting, Loose, Painful Dentures
Do you struggle with dentures that slip, cause soreness, or make it hard to eat your favorite foods? You're not alone — countless patients in Memphis and beyond face daily frustration from loose or poorly fitting dentures. If you’re tired of managing adhesives,...
Crown Lengthening for Restorative Success: Saving Damaged Teeth the Smart Way
A fractured tooth, a cavity extending below the gumline, or a crown that just won’t stay put can feel like a ticking clock for your smile. Fortunately, crown lengthening gives our team a strategic way to “buy space” and create a healthy foundation for long-lasting...
Zirconia vs. Titanium Implant Abutments: A Guide for Memphis Patients
When should Zirconia (Zirconium) Abutments be used? Dr. Gordon Christensen gives an excellent review of this recently published article.